The concept of “matter” underpins our understanding of the physical world, but have you ever wondered what matter truly is?
Is it just the solid objects we can touch, or does it go deeper, into realms invisible to the naked eye? How did ancient thinkers begin to explore this fundamental question, and how far have we come in our quest to define it?
While its meaning might seem obvious today, the journey to fully understand matter has taken thousands of years, filled with breakthroughs that reshaped our view of reality.
What is Matter?
At its core, matter is defined as anything that takes up space and has mass. This simple definition has served as the foundation for countless scientific investigations.
Scientists use a variety of key properties to better understand matter. Here are a few important ones:
- Density (ρ): Mass per unit volume ρ = m / V. Example: Water has a density of 1 g/cm³
- Specific Heat Capacity (c): Energy needed to raise 1 kg by 1°C Q = m × c × ΔT where Q is heat energy, m is mass, and ΔT is temperature change Example: Water’s specific heat is 4.18 J/(g·°C)
- Molar Mass (M): Mass of one mole of a substance M = m / n where m is mass and n is number of moles Example: Carbon’s molar mass is 12.01 g/molThese properties help us describe and predict how different types of matter behave.
The matter with which we interact on a daily basis—whether it is the air we breathe, the water we drink, or the solid objects we handle—is composed of atoms, the smallest units of chemical elements. However, our current understanding of matter extends far beyond this surface-level definition, delving into the atomic and subatomic levels.
Evolution of the Concept of Matter
Democritus and the Atomists (5th century BCE)
Democritus, an ancient Greek philosopher, first proposed the idea that matter is made up of indivisible particles. Democritus and his teacher Leucippus proposed that everything in the universe is made up of tiny, unchangeable particles known as “atoms” (from the Greek word “atomos,” which means “uncuttable“). He believed that these atoms moved through empty space and reacted in various ways to form different substances. Although this idea was not supported by experimental evidence, it laid the groundwork for later atomic theory.
Isaac Newton (17th century)
Sir Isaac Newton’s work in the 17th century helped to formalise the scientific concept of matter. His laws of motion and universal gravitation treated matter as having mass and the ability to exert forces, which marked a significant departure from earlier thinkers’ philosophical musings.
Newton’s concept of matter as a substance that could be affected by forces such as gravity influenced the development of physical sciences. However, while Newtonian physics provided a solid foundation, the nature of matter remained unknown.
The Periodic Table and the Chemical Nature of Matter (1869)
By the nineteenth century, scientific advances had elevated the study of matter to the level of chemistry. In 1869, Dmitri Mendeleev developed the Periodic Table after discovering individual elements, each of which is a distinct type of matter. This table arranged elements according to their atomic weight and chemical properties, revealing patterns that hinted at the underlying structure of atoms themselves. The periodic table was a significant step forward in our understanding of matter because it proposed that different substances were made up of simpler building blocks—atoms from various elements.
J.J. Thomson and the Electron (1897)
J.J. Thomson’s discovery of the electron at the turn of the twentieth century revolutionised our understanding of matter. Thomson’s experiments with cathode rays demonstrated that atoms are not indivisible and instead contain smaller negatively charged particles known as electrons. This discovery upended the long-held belief that atoms were the smallest unit of matter, paving the way for the complex atomic models we use today.
Thomson’s experiments resulted in the calculation of the electron’s charge-to-mass ratio e/m = 2V/(B²r²) where V represents the accelerating voltage, B is the magnetic field strength, and r is the radius of curvature of the electron’s path.
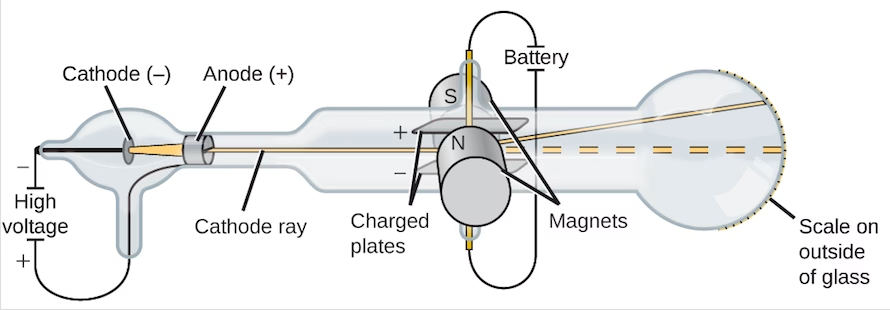
Thomson calculated e/m to be approximately 1.76 × 10^11 coulombs/kg. This value was significantly higher than for any known ion, implying that electrons were much lighter than atoms. Later experiments determined the electron’s charge (e) to be about 1.60 × 10^-19 coulombs, resulting in a mass of approximately 9.11 × 10^-31 kg.
Rutherford and the Nucleus (1911)
Ernest Rutherford’s gold foil experiment further revolutionized the concept of matter by revealing the existence of the atomic nucleus.
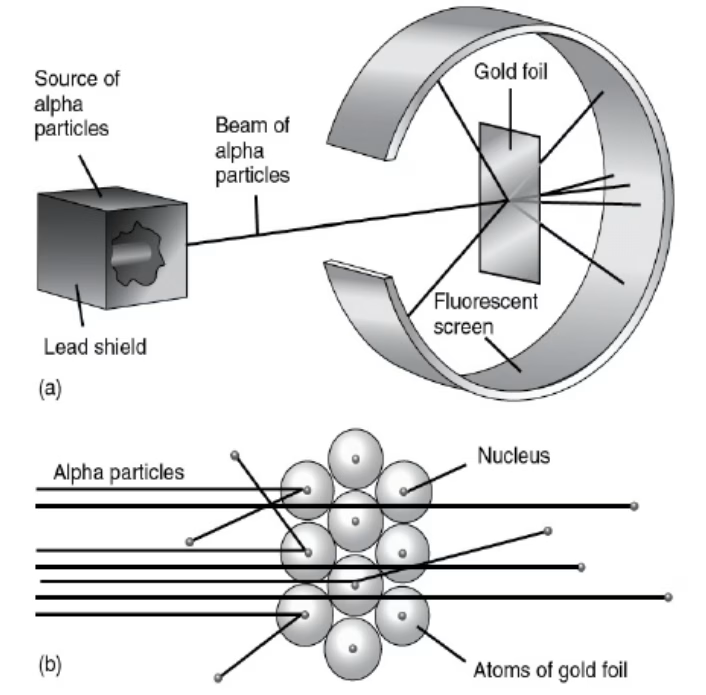
His findings showed that an atom’s mass is concentrated in a tiny central nucleus, surrounded by a cloud of electrons. This insight not only redefined atomic structure but also opened up new questions about the forces that hold these particles together.
The Standard Model
As our understanding of matter grew, scientists created the Standard Model of particle physics, which provides a framework for explaining the fundamental particles and forces that comprise the universe. This model regards quarks, leptons, and force carriers (such as photons and gluons) as the fundamental constituents of matter. Although a comprehensive theory, it is still incomplete, with many unanswered questions, particularly about gravity.
While the Standard Model helps to explain how particles interact, it is primarily a theory of subatomic particles. To gain a more complete picture of matter on a cosmic scale, we must first understand phenomena such as dark matter, which remains one of physics’ greatest mysteries.
The Standard Model is more than just a collection of particles; it is a mathematical framework that predicts how they interact. One of the most important equations in this model is the Dirac equation.
(iγ^μ∂_μ – m)ψ = 0
where: γ^μ are the Dirac matrices, μ represents the partial derivative of space-time coordinates, m is the mass of the particle and ψ the particle’s wave function
This equation describes the behaviour of spin-1/2 particles such as electrons and quarks, combining quantum mechanics and special relativity. It is fundamental to our understanding of matter.
The Role of Matter in Stars and Nuclear Fusion
Matter plays an important role in the life cycle of stars through the nuclear fusion process. In the cores of stars, hydrogen nuclei fuse to form helium, releasing enormous amounts of energy. Einstein’s famous equation E=mc^2 describes the fusion process, which converts matter into energy. This process is responsible for the light and heat emitted by stars, including our Sun. Nuclear fusion not only powers stars, but it also produces the heavier elements that comprise planets and, eventually, all known life.
The proton-proton chain reaction represents the process of nuclear fusion in stars. The initial step in this chain is:
¹H + ¹H → ²H + e⁺ + ν_e
This reaction releases energy using Einstein’s famous mass-energy equivalence formula:
E = mc² where: E is the energy released, m is the mass converted to energy, and c is the speed of light (about 3 × 10^8 m/s) For example, the Sun converts about 4 million tonnes of mass into energy every second, powering life on Earth.
Conclusion
From Democritus‘ atoms to the Standard Model, the concept of matter has undergone significant evolution. While we now have a thorough understanding of its atomic and subatomic structure, new discoveries continue to test and broaden our understanding. Matter, as we know it today, is both a fundamental building block of the universe and a complex topic of ongoing scientific research. Understanding it not only sheds light on the physical world, but also paves the way for future discoveries that could reshape our entire worldview.







